Introduction: Calaspargase pegol-mknl (CalPEG) was FDA-approved in December 2018 as a component of a multi-agent chemotherapeutic regimen for acute lymphoblastic leukemia/lymphoma (ALL/LLy) in pediatric patients and adolescent and young adults (AYA). It has recently been incorporated into national pediatric ALL consortia clinical trials. The pharmacokinetics and toxicity profiles were reported in two clinical trials (AALL07P4; Angiolillo et al, J Clin Oncol, 2014 and DFCI 11-001; Vrooman et al, J Clin Oncol, 2021), with similar safety profiles to historical pegaspargase (PEGasp). There is a paucity of published data regarding serum asparaginase activity (SAA) level variation or toxicity between CalPEG and PEGasp since FDA-approval. This is timely given the recent transition in asparaginase product availability, which may impact resource utilization, comprehensive drug safety monitoring, and clinical practice guidelines.
Methods: We conducted a single institution retrospective review of children and AYA with ALL/LLy who received an asparaginase product during upfront or relapse therapy between January 2022 to June 2023 as part of our chemotherapy quality and safety monitoring (Table). Patients received either PEGasp, CalPEG, and/or asparaginase Erwinia chrysanthemi recombinant (Rylaze) and had correlative SAA documented by a validated assay (NEXT Molecular Analytics; Chester, VA). The Common Terminology Criteria for Adverse Events version 5.0 was used to retrospectively grade asparaginase-related toxicities, including pancreatitis (≥ grade 3), venous thromboembolism (VTE; ≥ grade 2), hyperbilirubinemia (total bilirubin ≥ 5 mg/dL), allergy (≥ grade 3), and silent inactivation (SI, SAA < 0.025 IU/mL within 7 days post-treatment without allergic symptoms). Fisher's exact and Wilcoxon rank-sum tests were used to compare patient characteristics and toxicity incidence with pairwise post-hoc comparisons. P values were two-sided and considered significant if < 0.05. Generalized linear modeling (GLM) was used to model SAA over time and interactions among asparaginase product, toxicity, and immunophenotype.
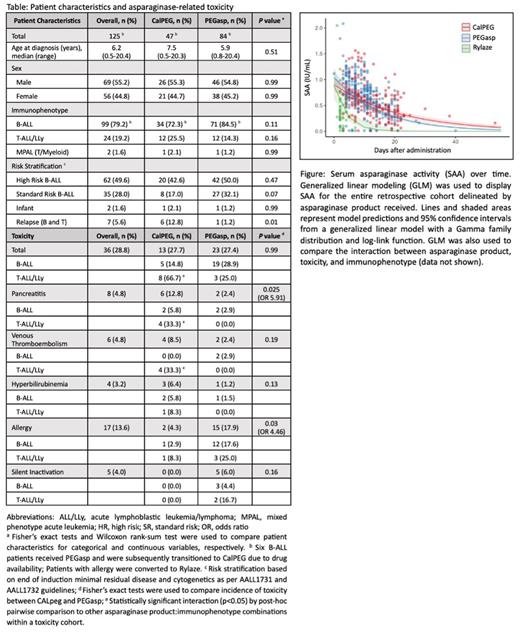
Results: One hundred twenty-five patients received either PEGasp, CalPEG, and/or Rylaze. (Table). Frequency of total asparaginase-related toxicity was comparable between CalPEG (27.7%) and PEGasp (27.4%). However, the incidence of pancreatitis was significantly higher in patients who received CalPEG (12.8%) compared to PEGasp (2.4%), P=0.025, with an Odds Ratio (OR) 5.91. T-ALL/LLy patients who received CalPEG had an increased incidence of both pancreatitis and VTE (33.3% each), with statistically significant pairwise interactions to PEGasp and/or B-ALL cohorts. Conversely, allergy was significantly higher in PEGasp (17.9%) compared to CalPEG (4.3%), P=0.03, with an OR 4.46. SI was found in 5.8% of PEGasp with none yet identified in CalPEG patients. The median peak SAA level over the first 10 days was not different among patients with adverse events; nor were median SAA levels higher in CalPEG patients, despite increased incidence in treatment-related toxicity (Figure). GLM fit slopes were predictably less negative in CalPEG compared to those of PEGasp; patients with toxicity had a larger modeled area under the curve. In 6 patients who were dose-capped to 3750 IU for body mass index (BMI) ≥ 40, all exhibited therapeutic SAA without any asparaginase-related toxicity.
Conclusions: To our knowledge this is the first single-institution post-market retrospective quality and safety analysis comparing CalPEG and PEGasp therapeutic drug monitoring and toxicity. Interestingly, the incidence of pancreatitis and VTE is significantly higher in patients receiving CalPEG and disproportionately higher in T-lineage. Median peak SAA levels were not predictive of toxicity, though GLM may help provide a predictive model. The rate of SI emphasizes the need for continued resource utilization of SAA monitoring. Our observed differences in CalPEG and PEGasp toxicity are discordant with two previously published clinical trials used for FDA approval of CALPEG (AALL07P4 and DFCI 11-001). Larger analyses of CalPEG SAA and toxicities are necessary to determine if these trends are reproducible. This information will help establish optimal utilization of SAA safety monitoring with the broader goal of identifying necessary practice changes in CalPEG clinical management.
Disclosures
Phillips:Merck Group: Consultancy. Rheingold:Abbvie: Other: Trial Steering Committee.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal